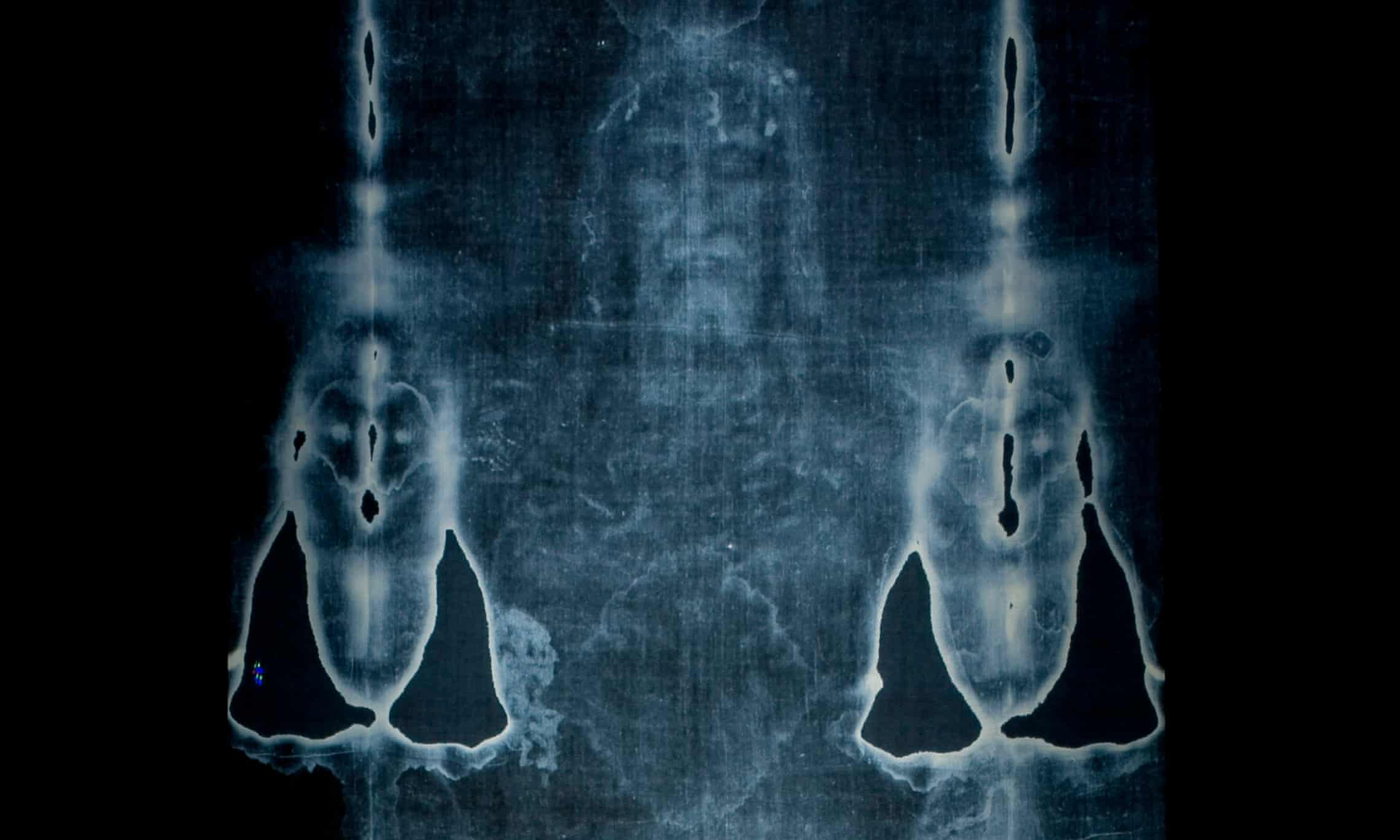
A photographic reproduction of the Turin shroud.
Photograph: Jorge Guerrero/AFP/Getty Images
The discovery that carbon atoms act as a marker of time of death transformed everything from biochemistry to oceanography – but the breakthrough nearly didn’t happen
Martin Kamen had worked for three days and three nights without sleep. The US chemist was finishing off a project in which he and a colleague, Sam Ruben, had bombarded a piece of graphite with subatomic particles. The aim of their work was to create new forms of carbon, ones that might have practical uses.
Exhausted, Kamen staggered out of his laboratory at Berkeley in California, having finished off the project in the early hours of 27 February 1940. He desperately needed a break. Rumpled, red eyed and with a three-day growth of beard, he looked a mess.
And that was unfortunate. Berkeley police were then searching for an escaped convict who had just committed several murders. So when they saw the unkempt Kamen they promptly picked him up, bundled him into the back of their patrol car and interrogated him as a suspected killer.
Thus one of most revolutionary pieces of research undertaken in the past century was nearly terminated at birth when one of its lead scientists was accused of murder. It was only when witnesses made it clear that Kamen was not the man the police were after that he was released and allowed to go back to the University of California Radiation Laboratory to look at the lump of graphite that he and Ruben had been irradiating.
Read the rest of this article...
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.